Aseptic Processing Contamination Control Strategy
Sterility is one major quality for medicines. Non-sterile products in the pharmaceutical industry could potentially harm the users. However, the degree of harm is dependent on the administration method and the number of microorganisms’ present. The immune system for the patient could also be a determinant.
For this sole reason controlling contamination in aseptic processing is very vital. It is especially crucial where the terminal sterilization has not been done. Aseptic processing involves the manufacture of drug products that do not go through the sterilizing step. With this method sterility is gained via putting off microbial ingress.
Steriline is one company that specializes in the manufacture of complete lines used in aseptic processing. The firm supplies aseptic processing machinery for pharmaceutical companies in the world. Formed back in 1989, the company has been producing top notch machines for aseptic manufacture. The company’s mission is to create distinct, durable and reliable machines that will leave no room for errors.
Steriline also aims at meeting the market demand in manufacturing consistent quality, improved efficiency machines and enhanced safety robotics. For all the aseptic manufacturing problems, visit Steriline and get your machine ready for work.
Sterility
Advancements in microbiology has really exemplified the contamination strategy which basically affects the understanding of sterility assurance and sterility. Studies have indicated that the human body has loads of microorganisms in different ecological niches. These organisms could be catalysts to diseases but they can only be discovered by cutting through genetic substances.
That only means that contamination assessment in the pharmaceutical industry is basically reliant on enumeration and recovery of microorganisms via culturing. Typically, this method justifies the pharmacopeia strategies for environmental monitoring and sterility testing. However, these tests are hindered by the microorganisms’ ability to remain metabolically active within the environment yet non-culturable.
There have been rapid sterility tests using the spectrophotometric technologies for environmental monitoring to give room for differentiation in the biologic particles and inert. However, these attempts are classified as emerging technologies rather than fully developed. These reasons call for the contamination strategy to be curated by the aseptic processing products manufacturers.
They will input more resources from their arsenal and they will focus more on contamination control rather than monitoring the entire process using methods that have their limitations.
Contamination Control Strategy
A contamination strategy is normally put in place for one major objective which is to provide sterility assurance. The strategy aims at producing sterile products in the pharmaceutical industry. In terminal sterilization, the sterility assurance level can give an understanding of potential non-sterility in the material. However, with aseptic processing, statistical assurance is not possible yet its aim is to stop any microbial ingress. It boils down to the manufacturer having a stringent and reliable contamination control plan to produce quality products that have sterility.
Aseptic Processing
It is producing sterile products via blow-fill-seal or conventional filling. In both methods, the specific item is sterile filtered inside a sterile holding container and then it is filled into depyrogenated vessel and sealed. To be able to achieve this while plotting out the contamination strategy, you need to consider some elements as discussed below:
Personnel Training and Gowning
While people could be the number one source of contamination in the entire production environment, training them on how they are supposed to work is very crucial. The operators need to be well-trained since they are the people who come into contact with the machines. The training should be continuous and intense covering the practical and theoretical aspects of the training. They ought to learn about hygiene and microbiology.
Good Facility Design
Sterile product manufacturing must be performed in classified cleanroom environments. That minimizes product contamination. Ensure you spend quality time is designing the appropriate layout for your facility. Ensure you have a short product flow path with airlocks in between the different grades of cleanrooms.
The perfect design should minimize contamination and have appropriate grade of cleanroom. You should also use the right construction materials and also check out the layout whether it favors easy material flow etc.
Cleaning and Disinfection
While you want to keep the environment as clean as possible, you can be sure contamination has a high probability of happening. That is why you are advised to clean regularly and disinfect the area. You could use two disinfectants, one could be a sporicide which destroys fungal spores and bacterial endospores. For companies with batch filling, it is advisable to disinfect before and after each batch has been filled.
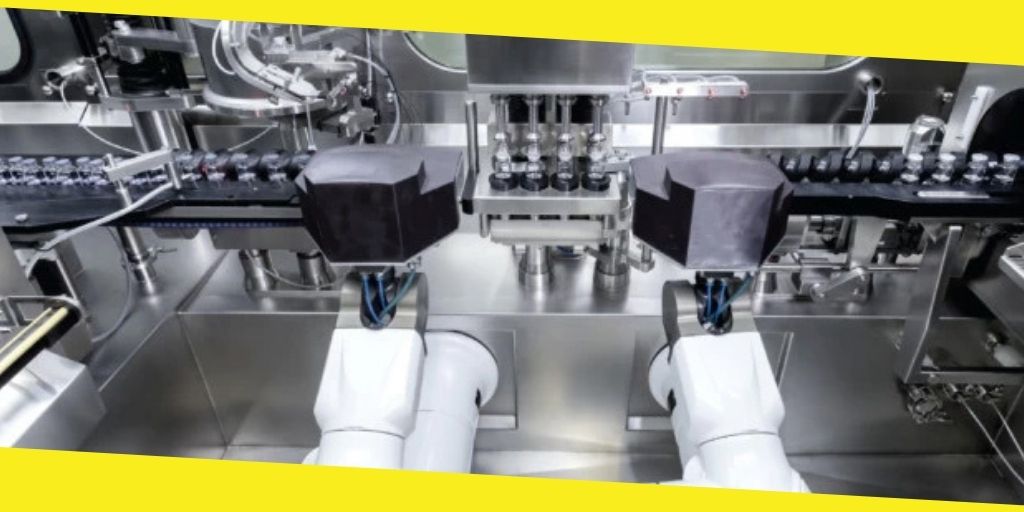
Robotics
The contamination control strategy does not exclude the robotics. Most of these are assembled and will probably be carried by humans to the area of operation. They should be cleaned and disinfected to avoid any contamination. Robotics can be more robust than humans and work faster. Steriline has a wide range of robotics for all your pharmaceutical needs. Order some of this brilliant robotics from them and ensure you apply the contamination control strategy too to have sterile products.
Contamination control involves a lot of crucial issues that a firm must take into consideration to ensure they produce products that are bacteria and fungi free. Apply the hacks mentioned and make your aseptic processing facility top quality with no contaminated products.
Recommended For You
What is Express Freight? Logistics Terms and Definitions
Most Inside
Most Inside offers high-quality recommendations and valuable updates to enhance all aspects of your life, providing premium guidance and enriching experiences.




